EvidentIQ group to launch new eCOA and eFeasibility offerings combining scientific services with a comprehensive software suite usable on any device
Breaking News:
Kathmandu Nepal
Dienstag, Apr 16, 2024

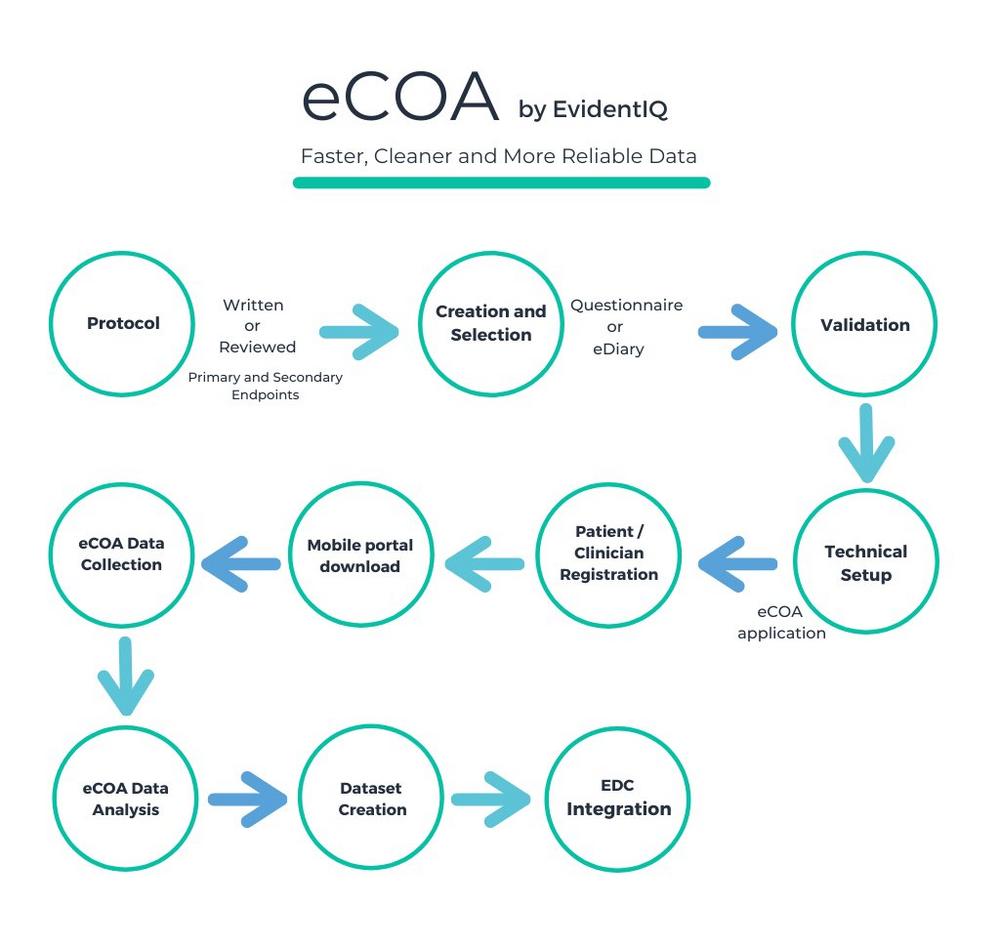
With its comprehensive eCOA solution, the new data science group, which has only recently been built by merging long-established providers XClinical, Carenity and Fortress Medical, has set out to fuse data science services and a scalable software platform. EvidentIQ customers benefit from an end-to-end eCOA package allowing to collect any patient / clinician / observer reported outcome data in a streamlined, compliant and efficient way. The solution can be used stand-alone or fully integrated into existing eClinical technologies, such as EDC systems. The EvidentIQ eCOA offering supports a bring-your-own-device (BYOD) policy and works on any iOS/Android phone.
Combining a software platform with scientific services
EvidentIQ’s combined offering of a platform with a scientific service offering attached to it empowers LifeScience professionals to conduct virtual and hybrid clinical trials. Unique eFeasibility solutions help optimize clinical trials and accelerate patient recruitment with a patient-centric approach.
EvidentIQ relies on Data Science and robust technologies to custom the design of clinical trials optimizing Product Profiles, Endpoints, Protocols, ICFs, and eCRFs. The package facilitates a complete digitalized patient enrollment journey for clinical trials from Multi-channel Pre-screening over eConsent Management to the Clinical Data Capture in the EDC.
Steady Market Growth for eCOA
Andreas Weber, CEO of EvidentIQ, points out the substantial market growth for Electronic Clinical Outcome Assessment:
“Most market analyses expect a CAGR of more than 15% for the eCOA market until 2027, and a global market volume of approximately 2.5 billion USD by then.”
When asked for the main drivers causing this growth Weber sees multiple developments besides the global Covid-19 pandemic.
“Keep in mind there is a high demand for clinical trials in emerging markets and consistently high R&D spendings in the pharmaceutical industry. Besides FDA and EMA are taking a tougher stance on COA data collection meeting regulatory quality guidelines.”
“Our new eCOA offering demonstrates perfectly how we create value for our customers and in doing so build a unique market position for our group. We empower life science companies of all sizes to transition from outdated, error-prone paper-based processes to digital data capture and data processing.”
EvidentIQ is a next generation technology-amplified data science group championing new standards in value creation and innovation driven relevance for customers. The EvidentIQ offering brings a pioneering end to end eClinical solution that meets increasing customer demand across clinical operations and clinical data management needs with a suite of applications within a single integrated cloud platform. By combining its platform with a broad data science service portfolio such as patient recruitment, patient engagement media and a host of RWE late phase solutions EvidentIQ significantly helps customers optimize HTA submissions, pricing and reimbursement needs.
EvidentIQ supports 15 of the top 20 pharma companies through novel RWE solutions and 150+ SMB customers in over 20 countries, including US, Germany, France, UK, Italy, Japan and China.
EvidentIQ Group GmbH
Rathausmarkt 5
20095 Hamburg
Telefon: +49 (89) 4522775000
Telefax: +49 (89) 4522775900
https://evidentiq.com
![]()