Concerted effort to ensure safe arrival of coronavirus vaccine
Breaking News:
Kathmandu Nepal
Samstag, Juli 12, 2025

The COVID-19 pandemic is demanding a fast response and expertise in handling medical products from packaging suppliers, service providers and equipment manufacturers. The companies involved have been able to quickly respond to these urgent needs through their packaging concepts for vaccines, PPE and medical devices. As they also had to get all this off the ground without physical contact with their customers there has been a significant increase in virtual collaboration. In association with the ramping up of production for well-established packaging like glass vials, developers from the sector also devised innovative solutions for counterfeiting prevention, serialisation, and temperature-controlled shipping packages.
In conjunction with vaccine manufacture, pharmaceutical companies relied on the expertise of experienced packaging providers and rapidly signed agreements to make sure that the distribution of the vaccine would not come unstuck due to a lack of packaging. Thanks to this boom in demand, the global market for pharmaceutical packaging equipment has experienced an unprecedented boost. “Pharmaceutical Packaging Equipment – Global Market Trajectory & Analytics”, a report published by ResearchAndMarkets, assumes an increase in growth for the sector worldwide of 13.2% in 2020. This will also benefit service providers undertaking the fill-and-finish process for the vaccines. In this conjunction, pharmaceutical companies also concluded a large number of contracts with service providers. Hameln-based company Siegfried, for example, is one of the facilities involved in the production of the BioNTech/Pfizer vaccine. IDT Biologika, located in Dessau-Roßlau, will be carrying out the fill-and-finish process for the viral vector vaccine developed by the DZIF (German Centre for Infection Research).
Specialists in the starting blocks
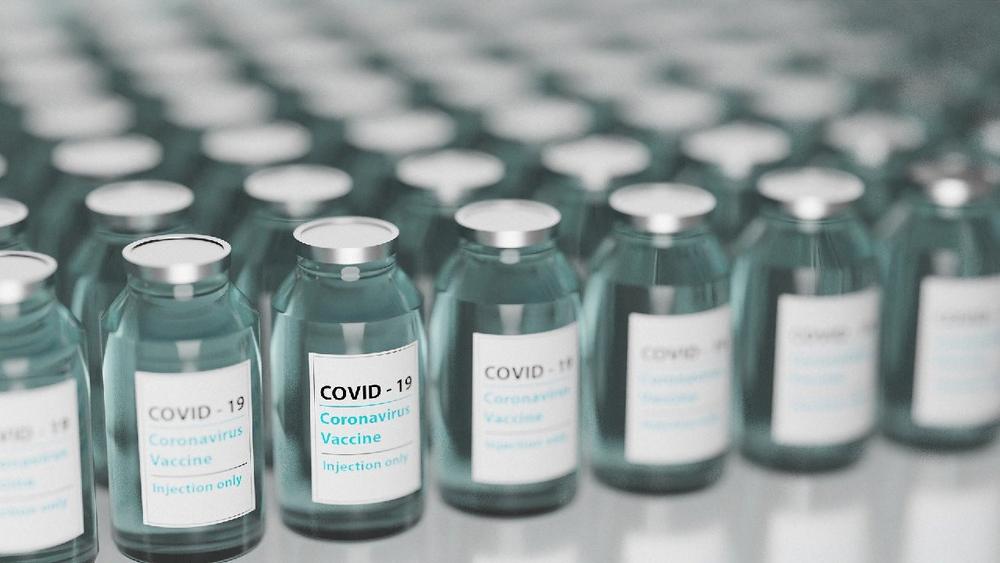
Most COVID-19 vaccines will be filled into borosilicate glass vials, from which they are then administered. In the pharmaceutical industry, this type of glass has been the standard packaging for vaccines for many years. As a result of this experience and the existing infrastructure, the major manufacturers can respond quickly to the high demand. In June 2020, for example, US vaccine manufacturer Moderna announced a partnership with Catalent to fill and finish the vials for the first batch of 100 million doses of vaccine at Catalent’s US plant in Indiana. German glass specialist SCHOTT is also involved in three-quarters of COVID-19 vaccine projects, according to market research company Global Data. The company has already produced and delivered millions of vials for the battle against COVID-19. Gerresheimer and the Stevanato Group are also producing pharmaceutical vials made of special glass to supply vaccines and medicines to the vaccination centres to combat the coronavirus. Every year, each of these three companies produces billions of vials made from type 1 borosilicate glass.
As well as the packaging materials, the filling systems play a key role in safely packaging the vaccines in the volumes currently required. Harro Höfliger, for example, pulled out all the stops to be able to deliver its machinery as quickly as possible to Pfizer’s production facility in Belgium. The high-performance packaging line was completed in record time at Harro Höfliger’s production facility in Backnang, Germany. It enables the fragile glass vials to be packed into boxes which are then labelled and printed with the relevant details.
Unbroken cold chain imperative
Due to the different types of vaccine involved, the requirements for transport, storage and shipping may vary and temperatures of as low as -80°C may be required. Special insulated boxes and digital transport boxes have proven suitable for the safe, temperature-controlled transport of pharmaceutical products. These boxes, which use cold packs or dry ice for cooling, maintain the temperature in the required temperature range during transport and protect medical products from damage.
Vaccine manufacturer Pfizer has developed its own special thermal shipper. Using dry ice, the temperature in the shipper can be maintained at a consistent level over a 10-day period. GPS temperature-enabled trackers are used in the shipping container to constantly monitor the temperature. They measure and record the temperature and vaccine inventory in real time.
Despite the rushed development, packaging vendors have not lost sight of the need for sustainability. and have also developed sustainable, thermally insulating packaging made from renewable plant-based components or paper.
Other manufacturers are pursuing their sustainability goals by means of recycling systems for the shipping boxes, allowing the Pfizer boxes fitted with temperature sensors to be re-used several times, for example. The start-up Tec4med has also developed a high-performance thermal insulation solution for the temperature ranges -80°C, 2-8°C and 15-25°C and for running times of more than 100 hours. This solution is also based on an environmentally friendly and resource-conserving recycling system.
Despite the enormous challenges involved in filling, packing and delivering billions of doses of vaccine in an extremely short time span, pharmaceutical companies have been able to draw on proven solutions from experienced suppliers. FACHPACK exhibitors can supply the pharmaceutical industry and its service providers with suitable solutions and have demonstrated their capacity to respond quickly to the urgent demand.
About FACHPACK
FACHPACK is the European trade fair for packaging, technology and processing. Over a compact three-day schedule in Nuremberg, it presents its extensive range of solutions for the packaging process chain for industrial and consumer goods. With a unique trade fair portfolio from the segments of packaging materials and supplies, packaging auxillaries, filling and packaging machinery, labelling, marking and identification technology, peripheral packaging machinery and equipment, packaging printing and finishing, palletizing technology, intralogistics, and services for the packaging industry, FACHPACK is the No. 1 industry gathering for the European packaging market that attracts trade visitors from all packaging-intensive sectors: food, beverages, luxury food, pharmaceuticals, cosmetics, chemicals, health care, non-food, pet food, automotive, technical articles, medical devices and other consumer and industrial goods.
NürnbergMesse GmbH
Messezentrum
90471 Nürnberg
Telefon: +49 (911) 8606-0
Telefax: +49 (911) 8606-8228
http://www.nuernbergmesse.de
![]()